Innovative Excipients
Effer-Soda® is a highly stable mixture of surface modified sodium carbonate and sodium bicarbonate powder developed for use in pharmaceutical and nutraceutical application. Effervescent delivery systems can provide a number of advantages, including possible improved bioavailability and faster on set of therapeutic action.
These convenient dosage forms are fast dissolving, highly soluble, and stable. For example, effervescent tablets provide the ease of carrying a tablet while at the same time giving the bioavailability of ingesting a solution. For these reasons, the delivery form is popular for many pharmaceutical products such as antacids, analgesic, or cough / cold formulations and nutraceutical product such as multi-vitamin and calcium supplement formulations.
Sodium bicarbonate is a primary ingredient in effervescent tablets and powders. Together with an acid, such as citric acid or tartaric acid, sodium bicarbonate reacts in the presence of water to the produce carbon dioxide (CO2). Consequently, the major disadvantage of sodium bicarbonate is that, in the presence of minute amounts of moisture and acid, it immediately effervesces.
This reactivity can lead to formulation instability and premature effervescence on stability and storage. Also, additional steps to pre-dry the powder along with special equipment and processing conditions are required during manufacture in order to avoid premature effervescence.
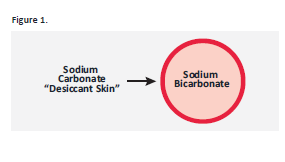
Effer-Soda® effectively protects against premature effervescence by reducing the moisture sensitivity of the formulation. This is accomplished by modifying the surface of the sodium bicarbonate with a desiccant skin of sodium carbonate that surrounds the sodium bicarbonate core.
This desiccant skin acts to protect the faster reducing sodium bicarbonate core by absorbing small amounts of moisture and thus reducing the possibility of premature effervescence in formulations. However, in the presence of large amounts of water, Effer-Soda®s readily dissolves and provides rapid effervescence.
Facts:
- Manufactured in a dedicated pharmaceutical facility under cGMP guidelines
- Free flowing highly stable powder
- Improves finished product stability by eliminating early effervescent reaction
- Provides rapid effervescence when desired in the presence of large amounts of water
- Reduces need for humidity controlled manufacturing Environment
- Reduces manufacturing steps by eliminating the need to pre-dry effervescent powder
- Supported by SPI Pharma global team of technical expert
Contact us for application engineering advice!
Pharmasperse® 416 is specifically engineered for the ready manufacture of orally dispersible powder (ODP) formulations intended for filling into sachets or stickpacks. These highly portable dosage forms offer an attractive alternative to tablet formulations, particularly for populations that may have difficulty taking solid oral dose medications (such as geriatric and pediatric patients). Pharmasperse is a versatile drug delivery system comprised of excipients that meet globally accepted compendia. This innovative platform is easy to use and offers a number of formulation advantages including:
Enhanced Functionality
Pharmasperse is designed to provide the functional properties required to formulate a high quality orally dispersible powder dosage form:
- Uniform particle size distribution helps to ensure drug uniformity form batch to batch.
- High density and low fines reduce the risk of a “puffing effect” that may lead to inhalation of powder.
- Excellent flow helps to prevent fill weight variation. Pharmasperse retains its excellent flow properties even in high drug loading formulations.
Rapid Speed to Suspension
The rapid speed of suspension of Pharmasperse helps to deliver fast therapeutic relief resulting in better patient outcomes.
Low Hygroscopicity
Pharmasperse is chemically stable and has low hygroscopicity. The low hygroscopicity helps to protect moisture sensitive active pharmaceutical ingredients (API) and can improve the stability of the formulation.
Excellent Organoleptics
- Pharmasperse provides a pleasant taste with a refreshing cooling sensation due to its negative heat of solution. This can help counter the poor taste of some APIs.
- The porous nature of Pharmasperse, along with its wetting characteristics, allow it to quickly disperse without agglomeration or over-drying in the mouth.
Streamlined Manufacturing Process
- Pharmasperse is a pre-formulated system that streamlines the development and manufacturing process.
- Less excipients needed in the formulation result in fewer ingredients to handle, test, and inventory.
- Manufacturing only requires dry blending with the API and the appropriate levels of sweeteners, flavors, and colors using standard processing equipment.
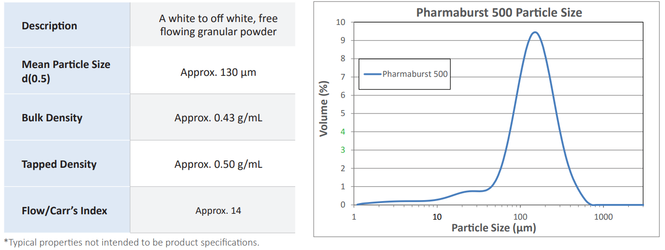
Typical Properties*
| Description | A white to off-white and free flowing granular powder |
| Mean Particle Size d(0,5) | Approx. 350 µm |
| Bulk Density | Approx. 0,6 g/ml |
| Tapped Density | Approx. 0,7 g/ml |
| Flow / Carr`s Index | Approx. 14 |
* Typical properties not intended to be product specifications.
Pharmaburst® 500
Pharmaburst® 500 is the global standard for orally disintegrating tablets (ODT). Its performance and versatility makes it an ideal choice among formulators to address many different pharmaceutical or nutraceutical product development challenges.
Global pharmaceutical manufacturers chose Pharmaburst technology because of its many benefits :
- Superior organoleptics
- Rapid disintegration
- Robust tablets
- High dilution capacity
Superior Organoleptic Quality
Pharmaburst is a co-processed platform comprised of excipients that meet globally accepted compendia. It has an optimum blend of polyols that deliver a smooth, creamy texture with a pleasant cooling sensation. It also has a neutral background flavor, allowing Pharmaburst to accommodate any desired flavor profile for customer satisfaction.
Compactibility
Due to the highly compactable nature of Pharmaburst 500, robust tablets can be made by direct compression employing relatively low forces. Manufacturing robust tablets at lower compression forces reduces the stress applied to multiparticulates such as taste masked actives, thereby ensuring that the integrity of the coating is maintained.
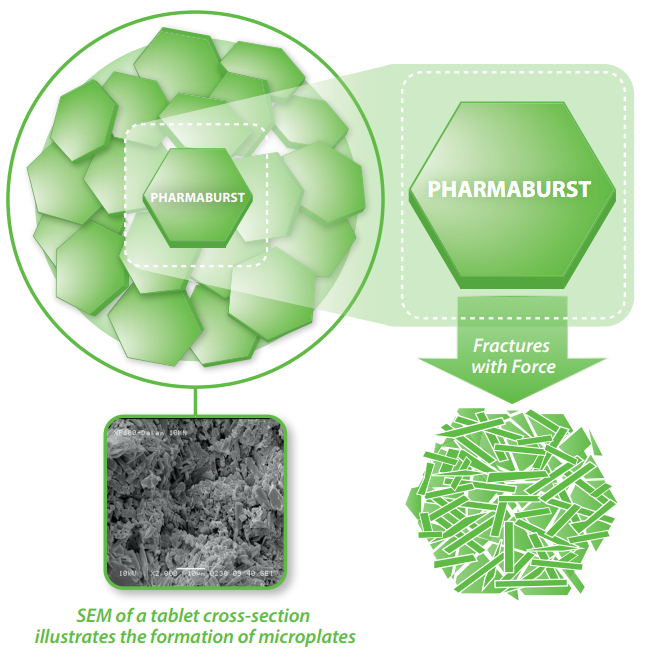
Mechanism of Action
When Pharmaburst is compressed, the co-processed granules break to form very small plate-like particles called microplates. These microplates make tablets formulated with Pharmaburst 500 highly porous resulting in rapid disintegration. The tight association between the microplates make Pharmaburst highly compactible and provides high drug loading capacity.
Rapid Disintegration
Fast disintegration time of 30 seconds or less is crucial for patient compliance with an orally dispersible tablet. Pharmaburst 500 produces fast disintegrating tablets that are hard enough to withstand the mechanics of production, storage, transportation, and being pushed out of a blister pack, yet it will disintegrate rapidly so the patient can swallow comfortably.
High Dilution Capacity
The compaction behavior of Pharmaburst 500 contributes to its ability to carry high drug loads. This is due to the co-processed granules fracturing around the drug particles during compression and filling in the void spaces.
Ease of Manufacture
Orally disintegrating tablets formulated with Pharmaburst are also easy to manufacture using standard direct compression equipment and processes. The active(s) are simply blended with Pharmaburst, flavors, sweeteners and colors. The lubricant is added in the final step and blended for the prescribed amount of time
Typical properties*