Good Practice
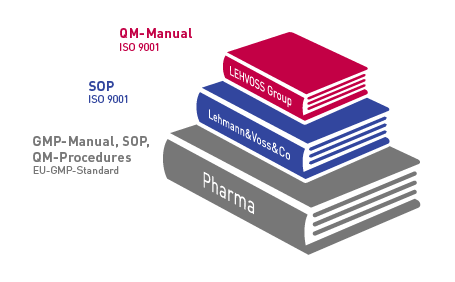
Pharmaceutical manufacturers operate in a strictly regulated environment marked by constantly increasing demands. Our customers can rely on being supplied by us with top quality raw materials which are available in the long term and safe.
Our processes comply with specifications laid down in written instructions. Regular certification and customer audits ensure an ongoing upgrading of this system.
As a result of our participation in expert meetings, symposiums and seminars, we are involved in continuing education on a regular basis and are hence a competent partner for our customers concerning the demands and conditions imposed by legislators, public authorities and users. Good Manufacturing Practice, Good Distribution Practice, Good Storage Practice – we deal with these and other relevant topics and implement the resulting demands promptly in our quality management system.